Researches Reviewed the Medical Records of 1436 People
Abstract
The clinical features and immune responses of asymptomatic individuals infected with astringent acute respiratory syndrome coronavirus two (SARS-CoV-2) take not been well described. Nosotros studied 37 asymptomatic individuals in the Wanzhou District who were diagnosed with RT–PCR-confirmed SARS-CoV-2 infections but without any relevant clinical symptoms in the preceding xiv d and during hospitalization. Asymptomatic individuals were admitted to the authorities-designated Wanzhou People'due south Hospital for centralized isolation in accordance with policy1. The median elapsing of viral shedding in the asymptomatic group was 19 d (interquartile range (IQR), 15–26 d). The asymptomatic grouping had a significantly longer duration of viral shedding than the symptomatic group (log-rank P = 0.028). The virus-specific IgG levels in the asymptomatic group (median Southward/CO, iii.4; IQR, ane.6–10.7) were significantly lower (P = 0.005) relative to the symptomatic grouping (median S/CO, 20.v; IQR, 5.viii–38.2) in the acute phase. Of asymptomatic individuals, 93.3% (28/30) and 81.one% (30/37) had reduction in IgG and neutralizing antibody levels, respectively, during the early on convalescent phase, as compared to 96.8% (30/31) and 62.2% (23/37) of symptomatic patients. Twoscore percent of asymptomatic individuals became seronegative and 12.9% of the symptomatic grouping became negative for IgG in the early convalescent stage. In add-on, asymptomatic individuals exhibited lower levels of 18 pro- and anti-inflammatory cytokines. These data suggest that asymptomatic individuals had a weaker immune response to SARS-CoV-ii infection. The reduction in IgG and neutralizing antibody levels in the early convalescent stage might take implications for immunity strategy and serological surveys.
Master
As of May 24, 2020, the coronavirus illness 2019 (COVID-19) pandemic, caused by SARS-CoV-2, has affected more than 5 1000000 people effectually the earth. Most patients with SARS-CoV-2 infections have reportedly had mild to astringent respiratory illness with symptoms such as fever, coughing and shortness of breath, which might appear 2–fourteen d after exposure. Nonetheless, there are other patients who are diagnosed by a positive RT–PCR test but are either asymptomatic or minimally symptomaticii,3,4,v,6. Increasing prove has shown that asymptomatic individuals tin spread the virus efficiently, and the emergence of these silent spreaders of SARS-CoV-2 has caused difficulties in the control of the epidemic2,5. However, our agreement of the clinical features and immune responses of asymptomatic individuals with SARS-CoV-2 infection is express. Hither nosotros draw the epidemiological and clinical characteristics, virus levels and immune responses in 37 asymptomatic individuals.
Results
Demographic characteristics
On Feb 6, 2020, the National Health Commission of China updated the COVID-xix Prevention and Command Program (fourth edition) for the management of close contacts, emphasizing identification and quarantine of asymptomatic individuals1. To identify asymptomatic individuals, the Wanzhou District Centers for Disease Control and Prevention (CDC) then conducted all-encompassing RT–PCR screening for ii,088 close contacts under quarantine. Individuals with positive RT–PCR results then were screened by signal prevalence surveys carried out by the local CDC and symptoms assessments reported by clinicians. Of these, threescore individuals claimed no symptoms in the preceding 14 d, according to local CDC records, and were transferred to a government-designated hospital for centralized isolation. On admission, 17 individuals were excluded for mild or atypical symptoms based on symptoms assessments reported by clinicians; half dozen individuals who developed symptoms 4–17 d subsequently access were likewise excluded. Finally, 37 asymptomatic cases, defined as individuals with a positive nucleic acid test result simply without any relevant clinical symptoms in the preceding 14 d and during hospitalization, were included in this written report. A full of 178 patients with confirmed SARS-CoV-2 infections were identified in the Wanzhou District before April 10, 2020, as tracked by CDC surveillance systems. In this report, the proportion of patients with asymptomatic infections was twenty.8% (37/178).
For antibiotic detection and cytokine measurements, 37 sex-, age-frequency- and comorbidity-matched mild symptomatic patients were selected for comparing with the asymptomatic individuals (Supplementary Table one). 30-seven sex- and historic period-frequency-matched command individuals from the Wanzhou District with negative RT–PCR results for SARS-CoV-2 were also selected for cytokines comparison.
Of the 37 asymptomatic individuals, the median age was 41 years (range, viii–75 years) and 22 were female person. Twenty-8 individuals had a confirmed history of contact with an RT–PCR-confirmed patient with COVID-19, and nine were Wuhan residents or had a travel history to Wuhan before the onset of infection (Supplementary Tabular array two).
Radiologic and laboratory findings
A complete claret count, blood biochemistry, coagulation function, liver and renal role and infection biomarkers were measured upon access (Supplementary Table ii) to monitor the potential disease progression, according to the COVID-19 Treatment Guidelines (5th edition) from the National Health Commission of China7. Of the 37 asymptomatic individuals, three had lymphopenia and one had thrombocytopenia. Six individuals had elevated levels of alanine aminotransferase, and 11 had increased C-reactive protein levels.
Upon access, chest computed tomography (CT) scans showed focal ground-glass opacities in 11 asymptomatic individuals (xi/37, 29.7%) and stripe shadows and/or diffuse consolidation in 10 individuals (10/37, 27.0%), whereas 16 individuals (xvi/37, 43.2%) had no abnormalities (Fig. 1). Five individuals adult focal ground-glass opacities or stripe shadows on breast CT within five d of hospital admission. At that place were no pleural effusions, air bronchogram signs or enlarged lymph nodes, which were typical changes seen in critically symptomatic patients8,9,10. Abnormal radiological findings confined to one lung were identified in 66.7% (14/21) of the asymptomatic individuals, whereas 33.3% (7/21) had abnormalities in both lungs.
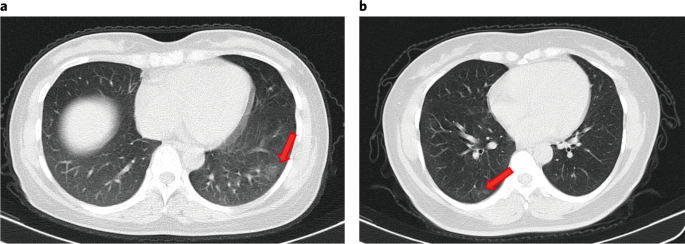
a, CT scan of a 45-year-old female showing focal ground-drinking glass opacities in the lower lobe of the left lung (arrow). b, CT scan of a l-year-old female showing footing-glass opacities and stripes coexisting in the lower lobe of the right lung (arrows).
Virological outcomes
We compared the RT–PCR cycle threshold (Ct) values of the first positive nasopharyngeal swabs for all 37 asymptomatic individuals and 37 symptomatic patients. The initial Ct values for 37 asymptomatic individuals and 37 symptomatic patients appeared similar (ORF1b 32.8 (IQR, thirty.9–35.8) versus 31.vii (IQR, 30.3–35.1), P = 0.336; N 32.6 (IQR, 29.5–34.6) versus 33.5 (IQR, 31.3–37.ii), P = 0.126) (Fig. 2a). The median duration of viral shedding, defined as the interval from the kickoff to last positive nasopharyngeal swab, in the asymptomatic individuals was 19 d (IQR, fifteen–26 d). The shortest observed elapsing of viral shedding was half dozen d, whereas the longest was 45 d. The median elapsing of viral shedding was 14 d (IQR, 9–22 d) in patients with mild symptoms. The asymptomatic group had a significantly longer duration of viral shedding than the symptomatic group (log-rank P = 0.028) (Fig. 2b). Notwithstanding, measurable virus shedding does non equate with viral infectivity, and further evaluation is needed to make up one's mind the respiratory SARS-CoV-2 viral load that is correlated with culturable virus11.
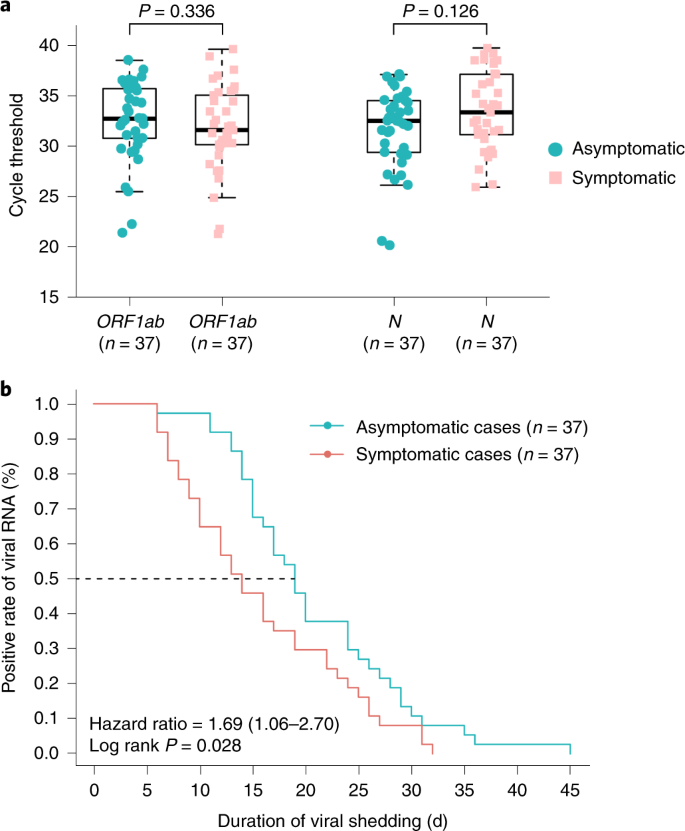
a, The Ct values of ORF1b and N obtained with RT–PCR that were detected in nasopharyngeal swabs from asymptomatic (northward = 37) and symptomatic (n = 37) groups. The box plots show the medians (middle line) and the get-go and 3rd quartiles (boxes), whereas the whiskers testify 1.5× the IQR to a higher place and below the box. Unpaired, ii-sided Isle of man–Whitney U exam P values are depicted in the plots, and the significant P value cutoff was set at 0.05. b, The Kaplan–Meier method was used to estimate the positive charge per unit of viral RNA, and the 2-sided log-rank test was applied to evaluate the significance deviation of the duration of viral shedding in the symptomatic and asymptomatic groups.
Source information
Virus-specific IgG and IgM in asymptomatic individuals
To investigate the acute antibody response to SARS-CoV-ii infection, virus-specific IgG and IgM were measured in serum samples from asymptomatic and symptomatic individuals. In the asymptomatic group, 81.i% (xxx/37) tested positive for IgG, and 83.8% (31/37) of the symptomatic group tested positive for IgG approximately 3–4 weeks later on exposure. Moreover, 62.two% (23/37) of the asymptomatic group were positive for IgM, whereas 78.4% (29/37) of the symptomatic grouping were IgM positive. Interestingly, IgG levels in the symptomatic grouping (median Due south/CO, 20.5; IQR, 5.8–38.2) were significantly higher than those in the asymptomatic group (median Southward/CO, 3.four; IQR, 1.6–10.vii) in the astute phase (the period when the viral RNA can be found in a respiratory specimen) (P = 0.005) (Fig. 3a).
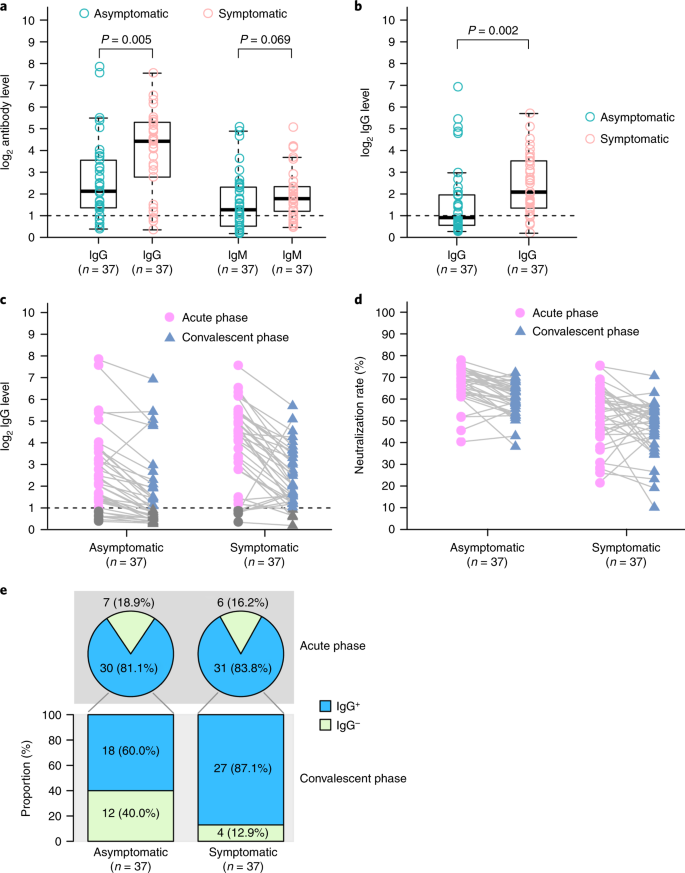
a, The comparing of virus-specific antibody levels in asymptomatic patients (n = 37) and symptomatic patients (northward = 37) with astute infections. b, IgG levels in patients with ambulatory-phase COVID-19 who were discharged from the hospital. c, Dynamic changes in virus-specific IgG levels in the astute and ambulatory phases. d, Dynamic changes in neutralizing serum antibodies in the acute and convalescent phases. Results are expressed as the average of two contained experiments. e, IgG-positive proportions of patients with COVID-nineteen in the acute and convalescent phases. The box plots in a and b show the medians (centre line) and get-go and third quartiles (boxes), and the whiskers show i.5× the IQR above and beneath the box. Unpaired, two-sided Mann–Whitney U test P values are depicted in the plots, and the pregnant P value cutoff was set at 0.05.
Source data
We too followed 37 asymptomatic individuals and 37 symptomatic patients into the early on ambulatory phase (8 weeks after they were discharged from the hospital). The IgG levels in the symptomatic group were nevertheless significantly higher than those in the asymptomatic group in the early on convalescent stage (P = 0.002) (Fig. 3b). Surprisingly, the IgG levels in 93.3% (28/thirty) of the asymptomatic group and 96.8% (thirty/31) of the symptomatic group declined during the early convalescent phase (Fig. 3c). The median percentage of decrease was 71.1% (range, 32.8–88.8%) for IgG levels in the asymptomatic group, whereas the median per centum of decrease was 76.2% (range, x.9–96.2%) in the symptomatic grouping. Using a pseudovirus-based neutralization assay (Methods), nosotros also observed a decrease in neutralizing serum antibodies levels in 81.1% (30/37) of the asymptomatic group and in 62.2% (23/37) of the symptomatic group. The median percentage of subtract was 8.3% (range, 0.5–22.viii%) for neutralizing serum antibodies in the asymptomatic group, whereas the median per centum of subtract was 11.7% (range, 2.3–41.1%) in the symptomatic group (Fig. 3d). Moreover, xl.0% (12/30) of asymptomatic individuals, only only 12.9% (iv/31) of symptomatic individuals, became seronegative for IgG (Fig. 3e).
Cytokines in asymptomatic individuals
To further elucidate the immune responses associated with SARS-CoV-2 infection, serum cytokines and chemokines levels were compared between the asymptomatic and symptomatic groups. Elevated concentrations of 18 pro- and anti-inflammatory cytokines were observed in the symptomatic grouping as compared to the asymptomatic group. Of these, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (P = three.39 × x−14), macrophage colony-stimulating gene (Chiliad-CSF) (P = five.08 × 10−xiii), growth-regulated oncogene-α (GRO-α) (P = 1.5 × 10−ten), granulocyte colony-stimulating factor (K-CSF) (P = 2.05 × x−9) and interleukin 6 (IL-6) (P = 6.33 × 10−9) showed the most significant changes (Fig. 4 and Extended Data Fig. 1). Moreover, the cytokines were further analyzed in the asymptomatic group and the 37 healthy controls. The plasma levels of 32 cytokines were like between the good for you controls and the asymptomatic individuals. Significantly higher levels of stem cell factor (SCF) (P = 1.48 × ten−9), IL-13 (P = iii.75 × x−7), IL-12 p40 (P = 7.08 × 10−6) and leukemia inhibitory factor (LIF) (P = i.33 × 10−3) were found in the asymptomatic group (Extended Data Fig. 2). Collectively, our information show that the asymptomatic individuals had a reduced inflammatory response characterized by low circulating concentrations of cytokines and chemokines.
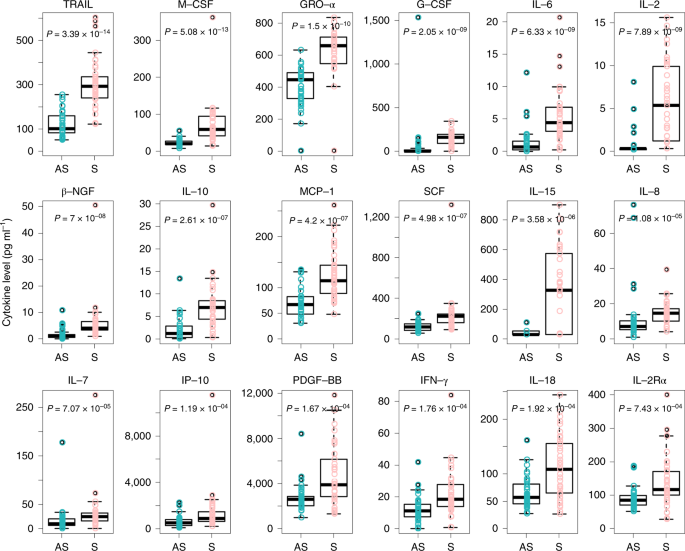
Samples from asymptomatic (n = 37) and symptomatic (due north = 37) patients with COVID-19 were collected in the acute stage during hospitalization, and assays were performed to measure out the concentrations of 48 cytokines and chemokines. The box plots prove the medians (eye line) and starting time and third quartiles (boxes), and the whiskers bear witness 1.5× the IQR above and below the box. Unpaired, two-sided Mann–Whitney U exam P values are depicted in the plots, and the pregnant P value cutoff was set at 0.001.
Source information
Discussion
The clinical features and allowed responses of asymptomatic individuals infected with SARS-CoV-ii have not been well described. Of the 178 laboratory-confirmed patients, 37 who never developed any symptoms throughout the disease course were included in this report. Our information showed that 20.8% of these patients had asymptomatic infections. However, this might not exist an accurate interpretation of the proportion of asymptomatic infections in the general population attributable to the fact that asymptomatic infections were identified from those who were at high take a chance for infection (including close contacts and individuals with a history of travel to Wuhan) and not from a random sample of people. Therefore, the proportion of asymptomatic infections needs to be determined through population screening12. Additionally, the proportion of asymptomatic infections might be even higher equally some cases might be missed by RT–PCR testing. Our group has successfully identified vii patients with SARS-CoV-2 infection from 148 cases with negative RT–PCR results and no symptoms by using an antibiotic test13. Therefore, timely RT–PCR and serological testing should be used in conjunction, which would benefit accurate estimation of the asymptomatic proportion. Still, serological testing has limitations, and tests vary in their specificity and sensitivity. Results might likewise be confounded past previously existing antibodies to SARS-CoV, MERS-CoV or common cold coronaviruses.
To appointment, the duration of SARS-CoV-2 RNA shedding has not been well characterized. In SARS-CoV, viral RNA was detectable in different specimens, including throat swabs, stool and urine, in more thirty% of patients for as long as 4 weeks after disease onsetfourteen. In MERS-CoV infections, viral shedding in respiratory secretions persisted for at least three weeks15. Recently, ane study of 191 patients with COVID-19 reported that the median elapsing of viral shedding was twenty d in survivors (range, 8–37 d)sixteen. In another report, the duration of viral shedding in nasopharyngeal aspirates was prolonged up to at to the lowest degree 24 d after symptom onset in xviii patients infected with SARS-CoV-two in Singapore17. In this study, the median duration of viral shedding in 37 patients with mild symptoms was 14 d, which was shorter than in previous reports. In comparison to symptomatic patients, the asymptomatic group had a significantly longer duration of viral shedding, with a viral shedding time of 19 d. Several factors might contribute to the variation of duration of viral shedding in different studies, including the severity of disease, definition of duration of viral shedding and frequency of specimen drove. Notably, detection of viral RNA does not necessarily hateful that infectious virus is present in respiratory specimens, and caution is required when applying virus shedding duration that was calculated based on RT–PCR to assess infection potential.
The force and duration of immunity after infection are key problems for 'shield amnesty'18 and for informing decisions on how and when to ease concrete distancing restrictions19,xx. Previous studies accept shown that circulating antibodies against SARS-CoV or MERS-CoV last for at least 1 year21,22. Sustained IgG levels were maintained for more than than ii years afterwards SARS-CoV infection23,24. Antibiotic responses in individuals with laboratory-confirmed MERS-CoV infection lasted for at least 34 months after the outbreak25. Recently, several studies characterizing adaptive immune responses to SARS-CoV-two infection have reported that near COVID-19 convalescent individuals have detectable neutralizing antibodies, which correlate with the numbers of virus-specific T cells26,27,28,29. In this study, we observed that IgG levels and neutralizing antibodies in a high proportion of individuals who recovered from SARS-CoV-2 infection kickoff to decrease within 2–three months subsequently infection. In another analysis of the dynamics of neutralizing antibody titers in eight convalescent patients with COVID-19, four patients showed decreased neutralizing antibodies approximately 6–7 weeks after disease onset30. 1 mathematical model also suggests a short duration of immunity after SARS-CoV-2 infection31. Together, these data might indicate the risks of using COVID-xix 'amnesty passports' and support the prolongation of public health interventions, including social distancing, hygiene, isolation of loftier-risk groups and widespread testing. Additional longitudinal serological studies profiling more symptomatic and asymptomatic individuals are urgently needed to determine the elapsing of antibiotic-mediated immunity. In addition, depression levels of anti-viral IgG in asymptomatic patients, who might exist more than likely to become seronegative, further back up the need for timely serosurvey to study the true infection rate.
Methods
Study pattern and participants
Between January 21 and February xix, 2020, 25 imported symptomatic patients (registered in Wanzhou District), who returned from Wuhan Metropolis or Hubei Province, were confirmed with SARS-CoV-two infection based on a positive RT–PCR test in Wanzhou District. (Wanzhou is the hardest-hit region of Chongqing City, which is a province-level municipality adjacent to Hubei Province.) Upwardly to April 10, 2020, a total of 2,088 individuals, including close contacts of confirmed patients and people returning from Wuhan, were placed under quarantine by the local CDC and tested by RT–PCR. Of these 2,088 individuals, 93 developed symptoms and tested positive for SARS-CoV-2.
On Feb 6, 2020, the National Health Committee of China updated the COVID-19 Prevention and Control Plan (4th edition) for the management of close contacts, emphasizing the identification and quarantine of asymptomatic individuals. The local CDC then conducted extensive RT–PCR screening of quarantined individuals. One time a case was confirmed positive by RT–PCR, the first prevalence survey was carried out by the local CDC staff. Individuals confirmed to have COVID-19 were asked to provide data, including demography (data of birth, gender and renal illness), preexisting conditions (including history of hypertension, diabetes mellitus, cardiovascular affliction, cerebrovascular affliction, chronic lung disease, renal illness, chronic hepatopathy and immunodeficiency diseases) and symptoms, likewise as screening records for the preceding 14 d (including fever, cough, expectoration, shortness of breath, chill, myalgia, sore throat, runny olfactory organ, breast distress, headache, diarrhea, vomiting and nausea). According to the COVID-nineteen Prevention and Command Plan (fourth edition), lx asymptomatic individuals based on preliminary screening were identified and transferred to a government-designated hospital for centralized isolation. On admission, i-on-one interviews were conducted by clinicians to corroborate the asymptomatic claims. Later on this screening, 17 individuals were excluded for mild or singular symptoms. Clinicians deport daily symptom screening once an individual is admitted to the hospital. Four to 17 days after admission, vi of these 43 previously asymptomatic individuals developed symptoms and were re-categorized equally symptomatic. Finally, 37 individuals with asymptomatic infections were informed about the study and consented to be included in this report.
Information technology was unclear whether individuals who present with asymptomatic infection might progress to clinical illness during the early on phase of the SARS-CoV-2 epidemic in China. Therefore, Chinese clinicians chose to administer interferon-alpha (IFN-α) inhalation, antiviral treatment (ribavirin orally) and supportive treatment (treatment to strengthen amnesty, such as thymopentin and Chinese medicine) to avert possible aggravation, according to the COVID-19 Handling Guidelines (5th edition) from the National Institutes of Health of China, published on February eight, 2020. The isolation and treatment strategy of asymptomatic infections should be updated according to official guidelines in different countries.
And so, 37 sex-, historic period-frequency- and comorbidity-matched symptomatic patients were selected for comparison with the asymptomatic individuals (Supplementary Tabular array 1). Thirty-seven sex- and age-frequency-matched controls with negative RT–PCR results for SARS-CoV-ii were besides included in this study. Individuals with lung, liver, kidney, cardiovascular, metabolic or immunodeficiency diseases were excluded.
Data collection
Epidemiologic, demographic, contact and exposure history, clinical presentations, breast CT, laboratory tests, treatment and outcome data were nerveless from inpatient medical records. Laboratory data collected for each patient included consummate blood count, coagulation contour, serum biochemical tests (including renal and liver function, electrolytes, lactate dehydrogenase and creatine kinase), serum ferritin and biomarkers of infection. Chest CT scans were done for all inpatients.
To identify SARS-CoV-2 infection, nasopharyngeal swabs were collected at least twice and tested by RT–PCR. RNA from all samples was isolated within 24 h. Viral RNA samples were extracted according to manufacturer instructions using the Nucleotide Acid Extraction Kit (DAAN Cistron, registration no. 20170583), which based on an automatic magnetic bead purification procedure. A commercial RT–PCR kit (DAAN Factor, registration no. 20203400063) was used for testing samples for SARS-CoV-2. Briefly, two target genes, including open reading frame1ab (ORF1ab) and nucleocapsid protein (N), were simultaneously amplified and tested during RT–PCR. Primers of RT–PCR testing for SARS-CoV-2 were co-ordinate to the recommendation by the Chinese CDC (ORF1ab forrad: CCCTGTGGGTTTTACACTTAA, ORF1ab contrary: ACGATTGTGCATCAGCT GA, ORF1ab probe: 5′-CCGTCTGCGGTATGTGGAAAGGTTATGG-3′ (FAM dye labeled); North forward: GGGGAACTTCTCCTGCTAGAAT, N opposite: CAGACATT.
TTGCTCTCAAGCTG, N probe: v′-TTGCTGCTGCTTGACAGATT-3′ (VIC dye labeled)). PCR cycling: 50 °C for fifteen min, 95 °C for 15 min, 45 cycles containing 94 °C for 15 s, 55 °C for 45 south (fluorescence collection).
Ct values less than 37 and greater than 40 were defined as positive and negative, respectively, for both genes. Samples with Ct values from 37 to forty were divers every bit inconclusive, and a 2d test was needed. Starting ane week after admission, nasopharyngeal samples were tested by RT–PCR every 2–iii d for the rest of the hospitalization menstruum. Patients with i positive RT–PCR result were divers every bit patients with SARS-CoV-2 infection. Patients with two consecutive negative RT–PCR results were defined as SARS-CoV-2 negative.
Definitions
A confirmed instance of COVID-19 was defined every bit an individual with nasopharyngeal swabs that were positive for SARS-CoV-2, using laboratory-based PCR. The symptomatic patients were divers as patients with laboratory-confirmed COVID-19 with symptoms such as fever, cough, sore pharynx and sputum. An asymptomatic instance was defined as an private with a positive nucleic acid examination issue but without any relevant clinical symptoms in the preceding xiv d and during hospitalization. A close contact was defined as (ane) anyone who had been within approximately six feet (2 meters) of a person infected with SARS-CoV-ii for longer than x min and (two) those who had direct contact with the infectious secretions of a COVID-19 patient. Close contact can occur while caring for, living with, visiting or sharing a healthcare waiting area or room with patients with COVID-19. The duration of shedding was calculated as the number of days from the first positive nasopharyngeal sample to the last positive sample based on RT–PCR testing. The last positive sample was followed past a negative RT–PCR issue on two sequential tests.
Detection of IgG and IgM against SARS-CoV-two
IgG and IgM against SARS-CoV-2 were detected in plasma samples using magnetic chemiluminescence enzyme immunoassay kits (Bioscience), according to the manufacturer'due south instructions. Briefly, recombinant antigens containing the nucleoprotein and a peptide (LQPELDSFKEELDKYFKNHTSPDVD) from the spike protein of SARS-CoV-two were immobilized on magnetic particles.
Neutralization detection using pseudovirus neutralizaion assay
A codon-optimized fasten (Due south) that lacked the C-terminal nineteen amino acids was used to generate a luciferase-expressing pseudovirus. The SARS-CoV-2 pseudovirus neutralization assay was carried out on 293T cells expressing ACE2 in a 96-well plate. Paired dilute sera (1:600) from an individual (one serum sample from the acute phase and another serum sample from the convalescent stage) were obtained, equal volumes of SARS-CoV-2 pseudovirus were added and the plates were pre-incubated at 37 °C for 1 h. Then, 293T cells expressing ACE2 were incubated with 100 μl of sera-pseudovirus mixture for 24 h. Three days later infection, the cells were lysed with 30 μl of lysis buffer (Promega) to mensurate the pseudoviral transduction. Relative luminescence units of luciferase action were detected using the Luciferase Assay Kit (Promega), according to the manufacturer'southward instructions. Experiments were repeated twice. The luciferase activeness was adamant by GloMax Microplate Luminometer (Promega). The neutralization rate (%) was calculated as following:
$${\mathrm{Neutralization}}\;{\mathrm{Rate}}\;{\mathrm{(\% )}}\;{\mathrm{ = }}\frac{{{\mathrm{RLU}}_{{\mathrm{pesudovirus}}} - {\mathrm{RLU}}_{{\mathrm{pesudovirus}}\;{\mathrm{with}}\;{\mathrm{serum}}}}}{{{\mathrm{RLU}}_{{\mathrm{pesudovirus}}} - {\mathrm{RLU}}_{{\mathrm{bare}}}}}100\%$$
Cytokines measurement
The sera of patients with laboratory-confirmed SARS-CoV-2 infections (asymptomatic group north = 37 and symptomatic group n = 37) were collected as early every bit possible during hospitalization. The sera of healthy individuals (north = 37) were included as control groups. The concentrations of 48 cytokines and chemokines were measured using the Bio-Plex Human Cytokine Screening Console (48-Plex no. 12007283, Bio-Rad) on a Luminex 200 (Luminex Multiplexing Instrument, Merck Millipore) post-obit the manufacturer's instructions. The 48 cytokines screening panel includes: fibroblast growth factor (FGF), eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), interleukin-1 beta (IL-1β), interleukin ane receptor adversary (IL-1RA), interleukin i alpha (IL-1α), interleukin-2 receptor-blastoff (IL-2Rα), interleukin-3 (IL-3), interleukin-12 p40 (IL-12 (p40)), interleukin-16 (IL-16), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-v (IL-5), interleukin-6 (IL-6), interleukin-7 (IL-vii), interleukin-8 (IL-8), interleukin-9 (IL-nine), growth-regulated oncogene-blastoff (GRO-α), hepatocyte growth factor (HGF), interferon alpha-ii (IFN-α2), leukemia inhibitory factor (LIF), monocyte-chemotactic protein iii (MCP-3), interleukin-10 (IL-10), interleukin-12 p70 (IL-12 (p70)), interleukin-xiii (IL-13), interleukin-xv (IL-15), interleukin-17A (IL-17A), interferon gamma-inducible protein (IP-10), monocyte chemoattractant protein-1 (MCP-i), monokine induced by gamma interferon (MIG), beta-nervus growth cistron (β-NGF), stalk cell factor (SCF), stem cell growth gene-beta (SCGF-β), stromal cell-derived factor i (SDF-1α), macrophage inflammatory protein-ane alpha (MIP-1α), macrophage inflammatory poly peptide-1 beta (MIP-1β), platelet-derived growth gene-BB (PDGF-BB), regulated upon activation, normal T cell expressed and presumably secreted (RANTES), tumor necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF), cutaneous T-jail cell-attracting chemokine (CTACK), macrophage migration inhibitory gene (MIF), TNF-related apoptosis-inducing ligand (TRAIL), interleukin-18 (IL-18), macrophage colony-stimulating cistron (M-CSF) and tumor necrosis gene-beta (TNF-β).
Statistical analysis
All continuous characteristics are described as the medians (IQRs), and categorical characteristics are described every bit numbers (%). Meaning differences of continuous characteristics between asymptomatic and symptomatic and asymptomatic and healthy control groups were determined by the Mann–Whitney U test. Categorical characteristics comparing was performed with Fisher's exact examination. P value cutoffs of 0.05 for antibody and 0.001 for cytokines indicated significance. The Kaplan–Meier method was used to analyze the duration of viral shedding in the symptomatic and asymptomatic groups. Statistical analyses were performed using R software (version 3.6.0).
Ethical approval
The study was approved by the Ethics Commission of Chongqing Medical University (reference no. 2020004). Written informed consent for participation in this written report was obtained from all adult participants or guardians on behalf of the children enrolled in this written report.
Reporting Summary
Further data on research design is available in the Nature Enquiry Reporting Summary linked to this article.
Data availability
Raw data in this report are provided in the Supplementary Dataset. Additional supporting data are available from the respective authors upon reasonable request. All requests for raw and analyzed information and materials will be reviewed by the corresponding authors to verify whether the request is subject to any intellectual holding or confidentiality obligations. Source data are provided with this newspaper.
References
-
COVID-xix Prevention and Control Plan, 4th edition (National Health Commission of the People'southward Republic of China, 2020).
-
Hu, Z. et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened amid close contacts in Nanjing, China. Sci. China Life Sci. 63, 706–711 (2020).
-
Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a written report of a family cluster. Lancet 395, 514–523 (2020).
-
Nishiura, H. et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-nineteen). Int J. Infect. Dis. 94, 154–155 (2020).
-
Bai, Y., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 323, 1406–1407 (2020).
-
Mizumoto, G., Kagaya, K., Zarebski, A. & Chowell, One thousand. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-nineteen) cases on board the Diamond Princess cruise ship, Yokohama, Nihon, 2020. Euro. Surveill. 25, 2000180 (2020).
-
COVID-19 Handling Guidelines, 5th edition (National Wellness Commission of the People'southward Commonwealth of China, 2020).
-
Xiong, Y. et al. Clinical and high-resolution CT features of the COVID-19 infection: comparing of the initial and follow-upwardly changes. Invest. Radiol. 55, 332–339 (2020).
-
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, Communist china: a descriptive study. Lancet 395, 507–513 (2020).
-
Wang, D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
-
Atkinson, B. & Petersen, E. SARS-CoV-2 shedding and infectivity. Lancet 395, 1339–1340 (2020).
-
Gudbjartsson, D. F., et al. Spread of SARS-CoV-2 in the Icelandic population. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2006100 (2020).
-
Long, Q. X., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. https://doi.org/10.1038/s41591-020-0897-1 (2020).
-
Xu, D. et al. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the ambulatory phase. Eur. J. Clin. Microbiol. Infect. Dis. 24, 165–171 (2005).
-
Oh, M. D. et al. Viral load kinetics of MERS coronavirus infection. North. Engl. J. Med. 375, 1303–1305 (2016).
-
Zhou, F. et al. Clinical grade and risk factors for bloodshed of developed inpatients with COVID-19 in Wuhan, Prc: a retrospective cohort study. Lancet 395, 1054–1062 (2020).
-
Young, B. East., et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323, 1488–1494 (2020).
-
Weitz, J. S., et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat. Med. https://doi.org/10.1038/s41591-020-0895-3 (2020).
-
Norheim, O. F. Protecting the population with immune individuals. Nat. Med. https://doi.org/x.1038/s41591-020-0895-iii (2020).
-
Phelan, A. L. COVID-nineteen immunity passports and vaccination certificates: scientific, equitable, and legal challenges. Lancet 395, 1595–1598 (2020).
-
Cao, W. C., Liu, W., Zhang, P. H., Zhang, F. & Richardus, J. H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 357, 1162–1163 (2007).
-
Choe, P. 1000. et al. MERS-CoV antibiotic responses ane yr afterwards symptom onset, South Korea, 2015. Emerg. Infect. Dis. 23, 1079–1084 (2017).
-
Guo, Ten., et al. Long-term persistence of IgG antibodies in SARS-CoV infected healthcare workers. Preprint at https://www.medrxiv.org/content/10.1101/2020.02.12.20021386v1 (2020).
-
Wu, L. P. et al. Elapsing of antibiotic responses after severe astute respiratory syndrome. Emerg. Infect. Dis. 13, 1562–1564 (2007).
-
Payne, D. C. et al. Persistence of antibodies against middle east respiratory syndrome coronavirus. Emerg. Infect. Dis. 22, 1824–1826 (2016).
-
Ni, L., et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity https://doi.org/10.1101/2020.03.17.20037713 (2020).
-
Thevarajan, I. et al. Breadth of concomitant immune responses prior to patient recovery: a case report of not-astringent COVID-19. Nat. Med. 26, 453–455 (2020).
-
Wu, F., et al. Neutralizing antibiotic responses to SARS-CoV-2 in a COVID-nineteen recovered patient cohort and their implications. Preprint at https://www.medrxiv.org/content/10.1101/2020.03.xxx.20047365v2 (2020).
-
Suthar, M. Due south., et al. Rapid generation of neutralizing antibody responses in COVID-nineteen patients. Preprint at https://www.medrxiv.org/content/ten.1101/2020.05.03.20084442v1 (2020).
-
Wang, 10., et al. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Preprint at https://www.medrxiv.org/content/x.1101/2020.04.xv.20065623v3 (2020).
-
Kissler, Southward. Thou., Tedijanto, C., Goldstein, E., Grad, Y. H. & Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 368, 860–868 (2020).
Acknowledgements
Nosotros thank C.-Y. Yang and B. Anderson for critical review of the manuscript. This study received the following funding: the National Science and Technology Major Projection (2017ZX10202203); the Emergency Project from the Science & Technology Committee of Chongqing; and the National Natural Scientific discipline Foundation of Red china (grant nos. 81871656 and 8181101099). We admit all patients involved in the study.
Author information
Affiliations
Contributions
Conceptualization: J.-F.Q., J.C. and A.-L.H. Investigation: Q.-X.L., X.-J.T., Q.-L.Due south., Q.L. and H.-J.D. Writing—original typhoon: Q.-X.L., H.-J.D. and J.C. Writing—review and editing: Q.-X.L., H.-J.D., J.C., Q.-L.S., J.-F.Q. and A.-L.H. Funding acquisition: J.C. and A.-Fifty.H. Resources: J.-Y., J.-L.H., W.Ten., Y.Z., F.-J.L., Thou.Southward., F.Z., J.Thou., B.West., X.-G.L. and J.-J.L. Supervision: A.-L.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Saheli Sadanand was the chief editor on this article and managed its editorial process and peer review in collaboration with the residual of the editorial team.
Publisher's annotation Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. ane Comparing of serum cytokine/chemokine concentrations between the asymptomatic and symptomatic groups.
Samples from asymptomatic individuals (due north = 37) and symptomatic patients (n = 37) were nerveless for assays measuring the concentrations of 48 cytokines and chemokines. 25 cytokines or chemokines were like between asymptomatic individuals and symptomatic patients. 5 cytokines or chemokines were significantly college in asymptomatic individuals than in symptomatic patients. The boxplots evidence medians (middle line), commencement and tertiary quartiles (boxes), while the whiskers testify 1.5× the interquartile range (IQR) above and below the box. Unpaired, two-sided Mann-Whitney U test p values are depicted in plots, and the significant p value cutting-off was set to 0.001.
Source data
Extended Data Fig. ii Comparison of serum cytokine and chemokine concentrations betwixt the asymptomatic and salubrious groups.
Samples from salubrious controls (due north = 37) and asymptomatic individuals (north = 37) were nerveless for assays measuring the concentrations of 48 cytokines and chemokines. A. Thirty-ii cytokines or chemokines were similar between healthy controls and asymptomatic individuals. B. Xvi cytokines or chemokines were significantly unlike between healthy controls and asymptomatic individuals. The boxplots testify medians (heart line), first and third quartiles (boxes), while the whiskers show 1.5× the interquartile range (IQR) above and beneath the box. Unpaired, two-sided Mann-Whitney U test p values are depicted in plots, and the pregnant p value cutting-off was set to 0.001.
Source information
Supplementary data
Source information
Rights and permissions
About this article
Cite this article
Long, QX., Tang, XJ., Shi, QL. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 26, 1200–1204 (2020). https://doi.org/x.1038/s41591-020-0965-6
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/s41591-020-0965-6
Farther reading
Source: https://www.nature.com/articles/s41591-020-0965-6